The Light Fantastic
Researchers use silk to create green optical technologies with potential applications ranging from bacteria detection to new solar cells
By Taylor McNeil
When Fiorenzo Omenetto ran into a colleague in the hallway of the Science and Technology Center in the summer of 2006, he couldn't have known that his research life was about to take off in an entirely new direction. And if you'd told Omenetto-an applied physicist by training and a self-described "femtosecond laser jock"-that it all would revolve around silk, he'd probably have laughed it off.
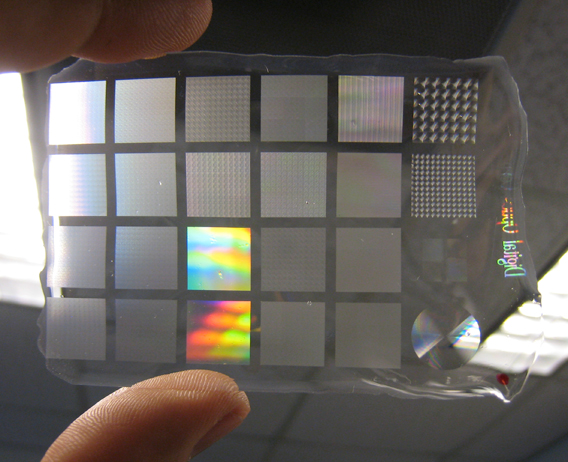
A demonstration sheet of the silk optical material showing different patterns. Photo: Courtesy of Fiorenzo Omenetto
But that's what happened when David Kaplan, a biomedical engineering professor, casually asked Omenetto, a newly hired associate professor in the same department, for help with a project he was working on. Kaplan wanted to know if a laser could make very tiny, precise cuts in a small piece of silk-based material he was molding for use as a replacement cornea.
Omenetto's first response on seeing the silk material-it looks like a thin film of transparent plastic-was, "Is this really silk?" It's a response that Kaplan is used to. People associate silk with ties and fancy clothing, and this was something of a completely different order.
But soon Omenetto was thinking that with the help of finely tuned lasers, and taking advantage of traditional molding techniques, patterns as tiny as a few tens of nanometers wide could be etched on the silk material. The implication hit him immediately: this could be used for optics. That's because visible light has wavelengths of between 400 to 700 nanometers, and with the silk able to maintain patterns at such small scales, it was an ideal medium for manipulating light.
Now Kaplan and Omenetto are thinking up all sorts of possibilities for the silk optics, from an ability to quickly detect harmful bacteria in food to new types of solar cells. Because silk is all natural and processed with water at room temperatures, the potential products would be clean and green-even implantable in the body or edible, if it came to that.
Butterflies and Nano-trees
Kaplan has been using silk in his research for some 20 years, engineering a range of human tissues, including ligaments, bone, cartilage and vascular systems. The silk, harvested from the cocoon of the mulberry silkworm, is one of the strongest fibers in the world.
To make the silk into optic material, the scientists take regular silk thread, boil it down to purify the protein in it and pour it in a mold. "We wait for it to crystallize and dry, and we peel it off and use that," Omenetto says.

A hologram with the Tufts logo created using the silk optical material. Photo: Courtesy of Fiorenzo Ormenetto
How would an application work? Omenetto points to an iridescent blue butterfly mounted behind clear plastic on his office wall. Some butterfly wings, he says, don't have pigment to reflect the colors in light, unlike, for instance, a shirt that's dyed with chemicals. Instead, the butterfly has "a forest of nano-trees, which captures white light, filters and selects it and reflects the blue color," solely because of the structure, he says. It's called structural color.
"So you can imagine if you flood the forest, you change the color of the wing," Omenetto says. He swivels his chair to face his computer monitor, and pulls up a short video. It shows a few drops of acetone being dripped on a blue butterfly specimen. As the liquid spreads, the color of the wings changes quickly to green. By flooding the forest of nano-trees, the environment around the trees has changed, and with it, the color we see. But soon a hand appears, fanning the wing, quickly evaporating the acetone, and presto, the wing changes back to its original blue color.
Using this idea, one theoretical application for the silk optics would be to determine if food products have been tainted by bacteria. A silk optic material would detect the presence of the bacteria, and change colors if it were present. And because it is made of silk, it wouldn't matter if the sensor was accidentally consumed along with the food product, say in a bag of store-bought spinach.

The source of it all: a mulberry silkworm cocoon.
The silk optic material would be patterned as a series of nanoscale peaks and troughs, with something that would react to the bacteria inserted into each trough. If the bacteria were present, the troughs would fill-and like a butterfly wing when its structure is altered-change colors, signaling the presence of bacteria. "The optical properties change depending on the biological activity of what is inside the optical material," Omenetto notes.
Silk optics have other potential uses. Components like enzymes or proteins could be mixed in with the liquid silk solution and used as biological markers for, say, oxygen or pH levels. When a component is added to the silk as it is drying, the silk locks the component into its structure, and within the hardened element, the enzyme or protein retains its function, says Omenetto. "The avenues of activation of the optics are the astounding thing."
As an example, Omenetto pulls out a sheet of the silk film, with splotches of red blood sealed inside. "This is starting to brown a little, but you can see there's quite a bit of red," he says, meaning that the blood is still fresh, all the more amazing considering it has been there for almost a year. Something like this could be used as an oxygen sensor: if oxygen is present, the human hemoglobin would change color.
"While we knew we could generate clear silk films," say Kaplan, "their quality with respect to optical sensors was very surprising."
The researchers have applied for patents for the technology, but there is still much work to be done and many more potential applications of the technology to explore. But one thing is certain: there's no shortage of silk to play with. "If you think of the amount of silk ties on the rack," Omenetto says with a laugh, "I think we're OK."
Taylor McNeil may be reached at taylor.mcneil@tufts.edu.

